Myoglobin is a protein found in the skeletal muscle tissue of animals. The most significant function of myoglobin is store oxygen.
Myoglobin exists in high concentrations in the muscle cells which allows organisms to hold their breath for a more extended period.
It is for this reason that diving mammals such as seals and whales have more myoglobin than humans do. In humans, the protein is only found in the bloodstream after a muscle injury.
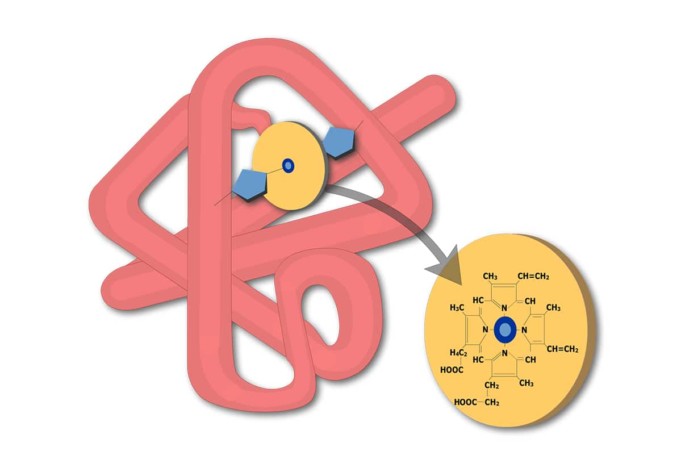
Myoglobin is related to hemoglobin, another oxygen-binding protein present in red blood cells. Both proteins contain a molecule called heme which gives them to bind oxygen reversibly.
The heme molecule contains iron which provides the protein with a reddish-brown color. However, the bond between hemoglobin and oxygen is more complicated than that between myoglobin and oxygen. As a result, hemoglobin is both able to store oxygen as wells as transport it.
Perhaps owing to its simplicity, myoglobin was the first protein to have its structure revealed by an X-ray crystallography in 1958 by John Kendrew and his associates. Kendrew later shared a Nobel Prize in Chemistry with Max Perutz in 1962 for this discovery.
Structure of myoglobin
Myoglobin consists of a single polypeptide chain of 150 amino acids with a single site to bind oxygen to it. Unlike hemoglobin, myoglobin does not bind cooperatively with oxygen.
Myoglobin is mostly present in the striated muscles of vertebrates. It can also be found at lower concentrations in endothelial, smooth muscles, and tumor cells. The MB gene encodes myoglobin in humans.
Myoglobin can also exist in the forms carboxymyoglobin (MbCO), oxymyoglobin (MbO2), and metmyoglobin (met-Mb).
Myoglobin has been obtained in pure crystalline form from many sources. It has a molecular weight of 16,700, which is about a quarter that of hemoglobin. Though the heme portion of all myoglobins is the same, the protein portions vary considerably between species.
Functions of myoglobin
The essential function of myoglobin is to supply oxygen to the muscle. To do so, it releases its supply of oxygen to the mitochondria, the powerhouse that makes up the respiratory chain, thus helping the myocytes to meet their high energy demands.
Myoglobin serves as an oxygen reservoir in muscles and a buffer of intracellular oxygen concentrations. This concept can be attributed to the fact that diving mammals have 10 to 30 times myoglobin compared to non-diving mammals.
The protein has also been shown to have enzymatic functions. Myoglobin is necessary for the decomposition of nitric oxide to nitrate. The removal of nitric oxide from the blood enhances mitochondrial respiration.
When in contact with blood from the veins, oxygen combines more readily with myoglobin than with hemoglobin. This favors the transfer of muscles from the blood to the cells.
Furthermore, myoglobin acts as an agent for removing reactive oxygen species.
Differences between myoglobin and hemoglobin
Myoglobin and hemoglobin are both proteins that bind oxygen to a single heme group. However, myoglobin has only one globulin group, while hemoglobin has four.
Despite both proteins having similar heme groups, myoglobin has a higher affinity for oxygen than hemoglobin.
It is for this reason that both proteins are different; while hemoglobin has the ability to transport oxygen, myoglobin is mainly to store oxygen.
Myoglobin and diseases
Damaged muscle tissue contains a very high concentration of myoglobin which is often released into the bloodstream and filtered by the kidneys. The excess myoglobin is toxic to the renal tubular epithelium and may lead to acute kidney injury.
The myoglobin itself is not a toxin; it is the ferrihemate part that dissociates from the myoglobin in acidic environments such as acidic urine.
Myoglobin is an indicator of injury in the muscles, making it a potential signifier for heart attack in patients with chest pain.