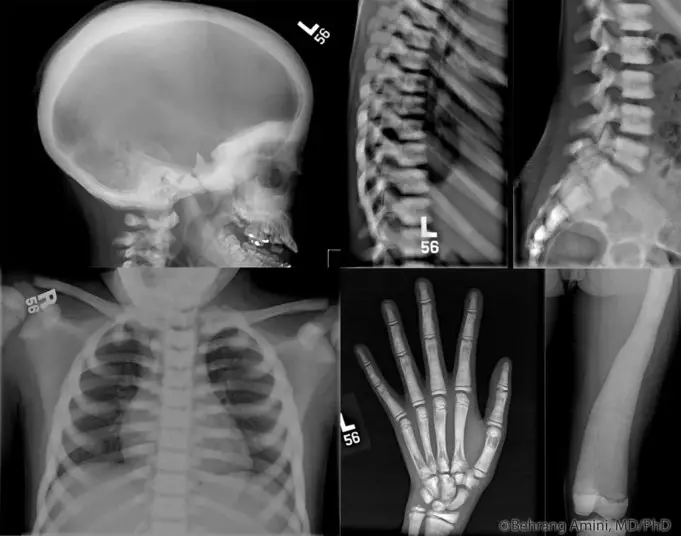
Marble bone disease goes by some other names such as Albers-Schonberg disease or Osteopetrosis. The medical term ‘Osteopetrosis’ was derived from the Greek phrases ‘osteo’ meaning bone and petros, meaning stone.
Marble disease is referred to as ‘Albers-Schonberg disease’, after a German radiologist who was the first to describe the medical condition in 1904.
Marble bone disease is a clinical group that comprises of genetically heterogeneous conditions that share the same symptoms of increased bone density when viewed through radiographs.
This rare inherited disorder is characterized by extremely dense, hard and brittle bones. Bones begin to have ‘stone-like’ quality but are abnormally brittle and fractures like chalk.
The skeletal system, like other systems of the body is well organized, made up of about 206 bones of different sizes, shapes and forms.
This wonderful stack of bones undergoes a constant metabolic process of breakdown and renewal; this always ongoing process of bone resorption and formation permits the skeleton to adjust to necessary modifications required for healthy growth, functioning, changes required for healing fractures and complex remodeling to maintain maximal bone strength.
Bones normally provides firm, non- brittle support. There are two major components of a bone, osteoid and mineral complexes.
Osteoid is a protein matrix that’s consist mostly of a fibrous protein called collagen while the mineral complexes comprise of crystals of calcium and phosphate, known as hydroxyapatite; that is embedded in the osteoid.
However, the major metabolic activities are carried out by osteoblasts, these generate the protein matrix, and ostroclasts, which are large multinucleated cells that digest and dissolve the components of the bone.
Marble bone disease or Osteopetrosis is a progressive disease that grows as long as the bone continues to grow; the bone density is increased due to the marrow cavities been filled with compact bone (refers to a dense bone that is solidly filled with organic ground substances and in organic salts, leaving only very tiny spaces).
This increase in bone mass, crowds the bone marrow resulting in a reduced amount of marrow; further reducing the capacity of the bone marrow to produce red blood cells.
Types of Marble Bone Disease
The Nosology Group of the International Skeletal Dysplasia Society classifies this increased bone condition into two types; it includes congenital form of marble bone disease and acquired form of marble bone disease.
The first type which is the Congenital forms of Marble bone disease is an inherited form of the condition characterized with a decreased number of osteoclasts (bone-reabsorbing cells) and decreased osteoclasts function. In this type of marble bone disease, fractures are frequent; deafness and impaired vision are common because of compression of the cranial nerves by extra-deposited bones in the skull which narrows the passageways of the cranial nerve.
This Marble bone disease is further classified based on the mode of inheritance: autosomal dominant, autosomal recessive and X-linked recessive.
Autosomal dominant type is the most common in this category; usually mild symptoms begin in late childhood or adolescence to adulthood appears in 6 in every 100,000 births and it may present with mild anemia or pathologic fracture or it can be completely asymptomatic (showing no symptoms).
The autosomal recessive form (also known as the malignant infantile type) appears soon after birth and frequently shortens life expectancy. In occurs in 1 of every 250,000-300,000 births. If left untreated, mortality rate can increase to 70% in 6 years with major deaths due to bone marrow failure; anemia, thrombocytopenia and infection.
Finally, the X-linked congenital form of marble bone disease is rarest, with only few known cases. There are also cases reported of an intermediate type of marble bone disease, comprising both milder autosomal recessive forms and dominant ones with early and severe onset.
On the other hand, Acquired Marble bone disease, is commonly as a result of fluoride deposition in the bone tissues (a condition termed fluorosis); this deposition of fluoride increases the densities of growing bones, but these bones are brittle (liable to break or easily to snap).
The extra poisoning of the bone is usually as a result of frequent long term consumption water containing excess minerals such as wee water or rocky water. Also, cancer patients can suffer from localized Marble bone disease, usually patients with breast cancer or prostate cancer, whose tumors have spread into the bone tissues.
Causes of Marble Bone Disease
In humans, mutations in at least 10 genes of the DNA have been identified to be the causative agent of Marble Bone Disease resulting in failure of osteoclast differentiation or function.
Another rare cause may be the increase of fluoride deposition in the bones, but the situation is so rare that it has remains a hypothesis.
The main manner and development of Marble Bone disease are best understood with reference to normal osteoclast formation and function.
| Condition | Inheritance | Gene | Mutation mechanism | Protein in affected gene |
| Osteopetrosis, severe neonatal or infantile forms | AR (Autosomal Recessive) | TCIRG | Loss of function | Subunit of V-ATPase pump |
| AR | CLCN7 | Loss of function | Chloride channel | |
| AR | OSTM1 | Loss of Function | Osteopetrosis associated trans-membrane protein | |
| AR | RANKL | Loss of function | Receptor Activator for Nuclear Factor KB Ligand | |
| AR | RANK | Loss of function | Receptor Activator for Nuclear Factor KB | |
| Osteopetrosis intermediate form | AR | CLCN7 | Chloride channel | |
| AR | PLEKHM1 | Loss of function | Pleckstrin homology domain containing family M, member 1 | |
| Osteopetrosis with renal tubular acidosis | AR | CAII | Loss of function | Carbonic anhydrase II |
| Osteopetrosis, late onset from (Albers-Schonberg disease) | AD | CLCN7 | Dominant negative | Chloride channel |
| Osteopetrosis with ectodermal and dysplasia and immune defect | AD | IKBHG (NEMO) | Loss of function | Inhibitor of kappa light polypeptide gene enhancer, kinase |
- RANKL: the mutation in this gene and seven others of its families leads to failure in osteoclast differentiation which has account for the rare osteoclast-poor forms of Autosomal Recessive Marble bone disease.
- CLCN-7 and TCIRG1: Homozygous mutations of these genes encoding the subunit of V-ATPase (TCIRG1) and the chloride-specific ion channel (CLCN-7) produce severe malignant Osteopetrosis phenotypes in humans. TCIRG-1 mutations are responsible for autosomal recessive Osteopetrosis (ARO) in more than 50% of patients because of the crucial role of V-ATPase in osteoclast function. While CLCN-7 plays a role in lysosomal acidification, which explains the severe neuronal storage and neurodegeneration in the central nervous system and retina in ARO patients.
- CAII: This gene plays a key role of replenishing expended protons and chloride ions in order to avoid alkalization of the blood in the kidney. The mutation CAII results in ARO with tubular acidosis.
- PLEKHM1: This protein plays a critical function in vesicle trafficking and acidification and heterozygous mutations is closely associated with intermediate forms of Marble bone disease.
- IKBKG (NEMO): This gene initiates the cellular division of osteoclasts and cells of the blood. Mutation of this gene is implicated to be the primary cause of ARO variants associated with immune system dysfunction.
- OSTM1: This gene encoding a protein membrane OSTM (Osteopetrosis associated trans-membrane protein) is closely associated with CLCN-7 and mutation in the gene are found in a subset of ARO patients with neurological involvement.
Mutations in the above-mentioned genes accounts for about 70% of marble bone disease cases and research are still been conducted to determine the genes responsible for the reminder.
The area of Osteopetrosis research has benefited from the many naturally-occurring rodent models of the disease; though many of the mutated genes that cause defects that have been observed in rodents have not yet been noticed in humans and these are the targets for future research.
Symptoms of Marble Bone Disease
Most common Clinical presentations of Marble Bone Disease include:
- Autosomal Recessive Osteopetrosis (ARO): Impaired hematopoiesis (formation of blood cellular components; red, white blood cells, platelets and blood plasma) which may lead to thrombocytopenia, anemia and infection; Cranial nerve compression leads to impaired vision and sensorineural hearing loss.
- Autosomal Dominant Osteopetrosis (ADO): Non-traumatic fractures, cranial palsy, osteoarthritis of hip, mandibular osteomyelitis
Other symptoms include:
- Infancy
- Anemia or pancytopenia (a condition of low counts of all three types of blood cells)
- Hepatosplenomegaly (due to extramedullary hematopoiesis) and Jaundice
- Obstructive sleep apnea
- Rhinitis (chronic inflammation of the mucous membranes in the nose)
- Failure to thrive.
- Early death
- Childhood
- Neurologic: cranial nerve palsies involving optic, trigeminal, facial, acoustic and other cranial nerves
- Macrocephaly (increase in head size)
- Micrognathia (abnormally small jaw)
- Anemia
- Delayed psychomotor development.
- Osteosclerosis
- Eating difficulties and retarded growth
- Fractures
- Rickets (results from inability to maintain normal calcium-phosphorus balance in extracellular fliud)
- Renal tubular acidosis
- Hypophosphatemia (a disorder characterized by a low level of phosphate in the blood), elevated acid phosphatase.
- Adulthood
- Maybe asymptomatic.
- Short statue
- Carpal tunnel syndrome (numbness and tingling in the hand and arm caused by a pinched nerve in the wrist)
- Bone pain
- Dental abscess and Osteomyelitis
- Tumors: Non-Hodgkin lymphoma, leukemia, bronchogenic carcinoma
- Elevated levels of Serum creatine kinase brain isoenzyme and acid phosphatase.
- Enlargement of the liver and spleen
Diagnosis of Marble Bone disease
The major clinical diagnostic of marble bone disease largely depends on the:
The radiographic appearance of the bone(s). The features of the radiological scan as evidence of marble bone disease progression include:
- Bone modeling defects at the metaphyses of lone bones such as funnel-like appearance and characteristics lucent bands
- Diffuse sclerosis, affecting the skull, spine, pelvis and appendicular bones.
- “Bone-in-bone” appearance particularly in the phalanges and vertebrae.
- Focal sclerosis of the skull base. Pelvis and vertebral end plates (also known as “sandwich” vertebrae
- Blood Tests: In the absence of the above features, increased concentrations of the creatine kinase KB isoenzyme and tartrate resistant acid phosphatase (TRAP) can be helpful in taking diagnosis of Autosomal dominant Marble Bone disease. Also, blood tests for complete blood count with differential, calcium, parathyroid hormone, phosphorus, creatinine, 25-hydroxyvitamni D and lactate dehydrogenase. These measurements will determine the need for supplementation and for referral to specialists.
- Bone biopsy is also another technique used to diagnose and distinguish between osteoclast-poor and osteoclast-rich subtypes of ARO; however, this technique is invasive and rarely performed.
- Genetic Testing: This testing is used to confirm diagnosis and differentiate between different forms of marble bone disease, assisting in providing information regarding causes and likely response to treatment. This is available on clinical or research basis for many of the genes implicated in Osteopetrosis conditions.
- Prenatal diagnosis: Prenatal diagnosis is theoretically possible in families whom their genetic mutation have been identified, thereby allowing for adequate and positive reproductive decisions.
Once diagnosis of a Marble bone disease condition is made, it is very necessary to distinguish between the forms as they all have different causes, response to treatment and reoccurrence risk.
Treatment of Marble Bone Disease
There is no specific treatment for marble bone disease as at present, but the only established cure for autosomal recessive malignant infantile Osteopetrosis (a form of marble bone disease) is hematopoietic stem cell transplantation (HSCT), however it has not been successful in all cases.
The HSCT treatment procedure allows the restoration of bone resorption by replacing already abnormal osteoclasts with healthy donor-derived osteoclasts. An adequate and efficient genetic study is however required to determine whether HSCT is appropriate as not all mutations benefit from the transplant.
Gamma-1b (Actimmune/human interferon gamma) which was approved by the U.S Food and Drug Administration is not a cure, but a treatment meant to delay the disease progression in patients with severe malignant infantile marble bone disease.
Rich nutrition is very important for patients with marble bone disease, involving mostly the use of calcium and vitamin D supplements if tests indicate low levels of blood minerals.
Other treatments and management of marble bone disease are supportive and involves treating of symptoms. Genetic counseling is recommended for families which have this disorder.
Summary
Genetic therapy studies are ongoing for marble bone disease patients especially those who do not benefit from hematopoietic stem cell transplants (HSCT) or who are unable to find matching donors, but these studies are still in their experimental phases as clinical trials have not been confirmed yet.
References;
- L. Lyndon Key Jr., William L. Ries, in Principles of Bone Biology (3rd Edition), 2008
- Classification of osteopetrotic conditions; http://ojrd.biomedcentral.com/articles/10.1186/1750-1172-4-5/tables/1
- Disease of musculoskeletal system;http://www.sciencedirect.com/topics/medicine-and-dentistry/albers-schoenberg-disease
- Bone and Joints; http://www.pathologyoutlines.com/topic/boneosteopetrosis.html
- Marble bone disease; http://www.britannica.com/science/marble-bone-disease