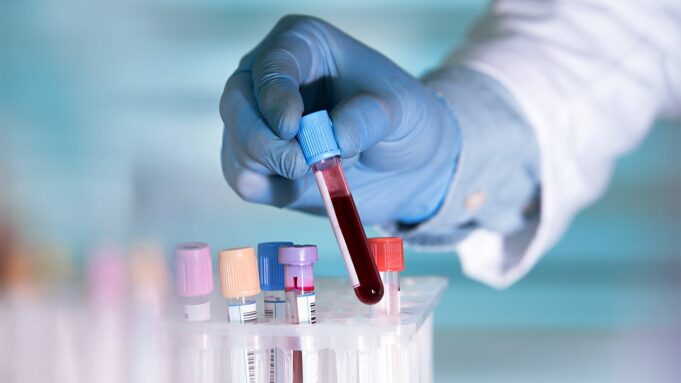
The sampling and evaluation of non-solid biological tissues, typically blood, is known as a liquid biopsy, also referred to as fluid or fluid phase biopsy.
This type of method, similar to a conventional biopsy, is primarily utilized as a diagnostic and surveillance tool for disorders such as cancer, with the additional benefit of being relatively non-invasive.
By obtaining repeated samples over a few weeks, liquid biopsy can also be used to confirm the efficacy of a cancer therapy medicine. Patients may find the equipment helpful in monitoring relapse after treatment.
Liquid biopsy is a blood test that detects circulating tumor cells and bits of DNA from the tumor cells in the blood.
The method can be investigated as a non-invasive and simple alternative to surgical biopsies. A liquid biopsy is a blood test that allows doctors to assess tumor-related information. It is meant to be used as a cancer early detection tool.
Recurrence and therapy monitoring and non-oncology applications, including prenatal screening, are other prospective cancer applications of liquid biopsy.
Could Liquid Biopsy be Better Than Conventional Biopsy?
When a person has a potentially malicious lump or symptoms, a doctor may perform a tissue biopsy, which is a process that collects cells for further analysis.
Under a microscope, the appearance of the cells can reveal whether cancer is present and what form of cancer it is and provide information about the patient’s prognosis. Furthermore, molecular examination of a tissue biopsy specimen can disclose information that can aid in developing a specific treatment plan.
Tissue biopsies that may include a large needle, an endoscopy instrument, or open surgery are intrusive, hazardous, expensive, and painful procedures necessary for patient care.
These tests can be impracticable to track tumors as they eventually develop and alter over time, making recurrent biopsies on a patient challenging. Nonetheless, they remain the gold benchmark for gathering information and detecting cancer.
However, scientists have been experimenting with a new method that could complement or perhaps replace tissue biopsies in some circumstances. The technique, known as a liquid biopsy, involves evaluating tumor material fragments, chemicals, and entire cells found in physiological fluids such as blood or urine.
Although there is a belief that liquid biopsy may substantially impact patient care in the future, most academics in the area acknowledge that the science behind the technique is still growing and that many concerns remain unsolved.
There has been a recent rise of interest in liquid biopsy tests that assess tumor DNA in the blood, known as circulating tumor DNA (ctDNA). Numerous ctDNA-based liquid biopsy procedures are currently in the phase of clinical trials.
As per BIS Research, the global liquid biopsy market is projected to reach $19.06 billion by 2032 from $2.50 billion in 2021 at a CAGR of 19.83% during the forecast period 2022-2032.
Using ctDNA for Early Detection and Monitoring of Cancer Patients
One possible application for ctDNA-based liquid biopsy is early cancer detection when treatment is most effective. It was observed that the tests occasionally yielded false-positive test results, and malignant DNA was discovered in some cases even though no cancer had grown.
Another issue is that these tests will uncover early-stage tumors that will either not develop at all or develop so slowly that they will never cause any damage to the patient.
The possibility of overtreatment is a key concern with early cancer detection and treating these slow-growing tumors could cause more harm than good.
The concept of diagnosing a patient solely through liquid biopsy is yet to be proven. It is still in its infancy and has a long road ahead.
There is also the possibility that ctDNA-based liquid biopsy could help guide precision medicine therapy by identifying specific molecular traits of a patient’s malignancy. Various studies have utilized liquid biopsy to identify ctDNA mutations that may be used to determine the best treatment.
Other research has shown that using ctDNA-based liquid biopsy to discover DNA alterations in patients’ malignancies on a wide scale is feasible.
ctDNA-based liquid biopsy may be beneficial for evaluating patients’ responses to treatment both during and after treatment since the biopsy is non-invasive and easy to repeat.
Clinicians anticipate that by tracking a patient’s response to the treatment, they will be able to make real-time modifications. In other words, if the test suggests that the treatment is not working, it could be halted or changed.
Conclusion
Liquid biopsy is an important aspect of medical decision-making since it helps acquire information for various treatment options. Liquid biopsy is an integral part of the precision medicine strategy because it ensures that the targeted treatments are used safely and effectively.
Due to prospects such as the introduction of informatics and technical advancements for a large client base, as well as the increased use of cancer and other illness diagnostic services, the liquid biopsy market is predicted to grow significantly.
Interested to know more about the growing technologies in your industry vertical? Get the latest market studies and insights from BIS Research. Connect with us at [email protected] to learn and understand more.