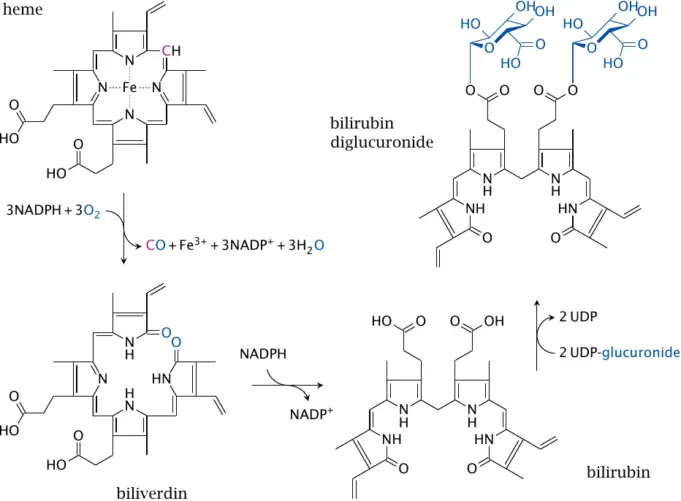
A heme is a ring-shaped, organic molecule, who due to its unique structure is capable of carrying, or holding an iron molecule.
A heme is made up of 4 small pentagon-shaped molecules made up of 5 atoms (4 carbons and 1 nitrogen) called a prryole. Four pyrroles together form a tetrapyrrole.
If the tetrapyrrole has substitutions on the side chains which allow it to hold a metal ion, it is called a porphyrin. Hence, a heme is an iron-holding porphyrin.
The iron molecule in a heme is held firmly by the balanced incredible forces of the four nitrogen molecules. The nitrogen molecules all point toward the interior of the larger ring they establish.
The dual and single bonds which attach the pyrroles are arranged evenly, so that the electrons stay stable and the entire molecule id balanced. This makes it an aromatic molecule.
Hemes are used for two known reasons: to move oxygen and to store electrons. Organisms use the heme molecule, in complex with unique shaped proteins to transport oxygen and store electrons.
These special proteins, like myoglobin and hemoglobin, are formed to enable the heme complex release or hold oxygen at the appropriate times.
Heme is named after the Greek word for blood, which is where it was first recognized. The red color of blood is created by heme and iron ion interacting to assimilate other colors and only reflect red.
A fairly different outcome is seen in chlorophyll, which is a porphyrin complex used in photosynthesis. chlorophyll houses a magnesium ion, instead of iron ion and chlorophyll has different side chains in contrast to the heme group.
This produces the green color of plants, rather than the red and purple hue of blood.
Heme Structure
Like all porphyrins, heme has a base structure ring of four pyrroles. This base molecule, is seen rarely as an intermediate in nature, is known as porphin.
There are different forms of heme, which conform to the many functions it serves in an organism. Specific proteins use the irregular side chains to attach to, and they alter the properties of the heme. However, the base structure is always the same.
The numbers on the molecules indicate points in which the molecule may receive substitutions and be modified for a specific use. Differences in the side-chains affixed to carbons 3, 8, and 18 form the difference between some of the most common heme groups.
For example, myoglobin and hemoglobin both carry Heme B. Heme B disseminates oxygen, and the proteins it is attached to, to help it expel the oxygen at the appropriate time. While Heme A works in the electron transport chain as part of cytochrome C.
This implies that it is entailed in transporting proteins and catalyzing reactions. The only discrepancy between the two molecules are the side-chains attached at carbons 3 and 18 but the side chain on carbon 8 remains the same.
Heme Function
Heme has two known functions. It binds gases, such as oxygen, and carry them throughout an organism. Special proteins then force the heme to expel its oxygen at the appropriate time. A good example of a protein of this kind is hemoglobin.
Hemoglobin is found in all blood cells, bound to the cell membrane, exposing the heme group to the blood plasma. So, when the blood cells moves through the lungs, they bind up as much oxygen as the iron in the heme can control.
The blood cells then travel to several parts of the body, such as the muscles. These cells are energetically using up oxygen and releasing carbon dioxide as a byproduct. Carbon dioxide forms an acid in the blood plasma, reducing the pH of the blood.
Like all proteins, hemoglobin responds to changes in pH by altering its shape. This change in shape compels the oxygen off, of the heme complex, discharging the oxygen into the blood plasma.
The oxygen diffuses into the muscle cells, where it is attached by myoglobin and transported to the mitochondria to be utilized. Myoglobin also has a heme group, but it functions in a distinct way so that oxygen remains attached until getting to the mitochondria.
The second function of hemes is holding electrons and stimulating reactions in the electron transport chain, which happens in all organisms. During oxidative phosphorylation in the mitochondrial membrane, electrons must be passed through a series of reactions, which slowly draw out their energy before disposing them in water and carbon dioxide.
The energy gained is reserved in the bonds of the molecule ATP, which most living things use as a primary source of energy. The heme groups in these cytochromes are from those in hemoglobin, as they have varied functions and bind to varied proteins.
Diseases
Studies have discovered an association between high intake of heme sourced from red meat with colon cancer. Heme deficiency has also been linked with the cause of malaria caused by Plasmodium falciparum.