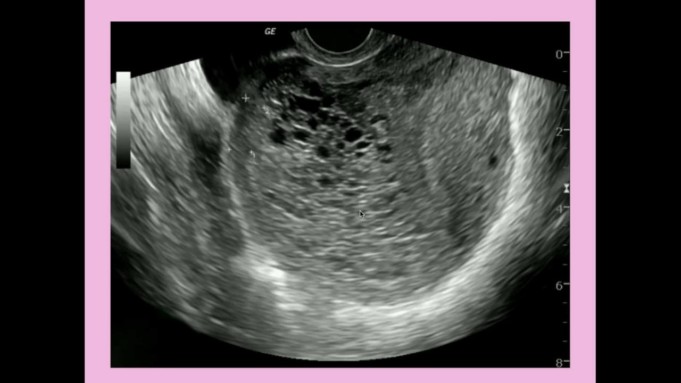
Gestational trophoblastic disease (GTD) is a disease terminology used to describe a group of rare tumors that develops during the early stages of pregnancy.
These groups of tumors are defined by abnormal trophoblastic proliferation. In preparation for pregnancy, the body produces trophoblasts; trophoblast is a layer of cells that surrounds a newly fertilized egg or embryo after conception.
This layer aids the embryo implant into the uterine wall. Trophoblasts also form a large part of the tissue that makes up the placenta (the organ that connects the developing fetus to its mother for nutrients).
In the case of Gestational trophoblastic disease, there is an abnormal change in the trophoblast cells that result in tumor development.
The gestational trophoblastic disease is very rare and can either exist in a non-cancerous (benign) or in a cancerous (malignant) form.
Other convenient medical terms may be used to describe these types of tumors because its original term- Gestational trophoblastic disease (GTD) is quite long and complicated.
The terms include:
- Trophoblastic tumor
- Trophoblastic disease
- Gestational tumor
- A gestational trophoblastic tumor (GTT)
- Molar Pregnancy
Gestational trophoblastic disease and other similar conditions have been known since ancient times but often been poorly understood.
The first description of a form of this disease was in 400BC, Hippocrates first described hydatidiform mole as dropsy of the uterus.
While in AD 600, Aetius of Armida described a uterus “filled with bladderlike objects”, which probably also represented this process. in 1700, Smellie first related the terms hydatid and mole, but not until 1827 that Velpeau and Boivin first recognized hydatids as cystic dilations of chorionic villi.
In 1889, Sanger invented the sarcoma uteri deciduocellulare as a malignant tumor derived from the decidua (a mucous membrane that lines the uterus and is modified during pregnancy or shed off during menstruation) of pregnancy.
In 1895, Marchand demonstrated these tumors to be the disease complication of pregnancy, abortion, or hydatidiform mole and described the proliferation of the syncytium and cytotrophoblast.
In 1903, Teacher confirmed Marchand’s work and sidelined Sanger’s theory of sarcomatous degeneration of the deciduas. Finally, Fels, Ernhart, Reossler, and Zondek demonstrated excessive levels of gonadotropic hormone in the urine of patients with Gestational trophoblastic disease.
The gestational trophoblastic disease is classified into two categories: Hydatidiform moles and Gestational trophoblastic neoplasia (GTN). Although Gestational trophoblastic disease begins in the uterus (womb), it behaves much differently from cancer of the womb.
The treatment approach is also different. Overall gestational trophoblastic tumors account for less than 1% of female reproductive system cancer.
Types and Causes of Gestational Trophoblastic Disease
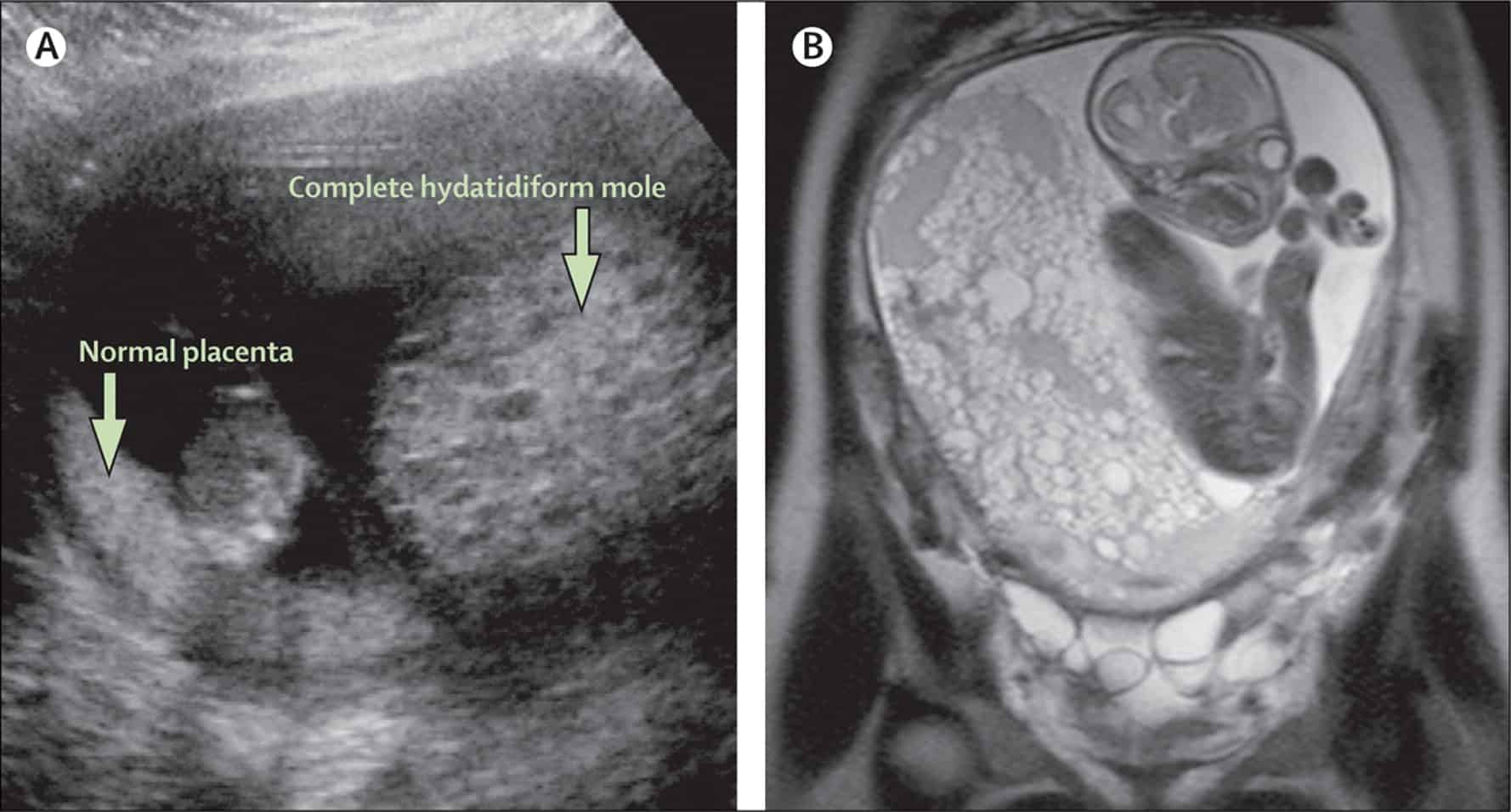
There are majorly two different types of Gestational trophoblastic disease, but five in total as the others are sub-classified under the two primary forms, they include:
Hydatidiform mole (partial or complete)
A hydatidiform mole is also referred to as molar pregnancy. In this type of Gestational trophoblastic disease, there is a problem with the fertilized egg; and there is an over-secretion of trophoblast tissue.
The excess trophoblast tissue grows into abnormal masses that are usually benign but can in some cases become cancerous.
There are two subdivisions of hydatidiform moles:
- Partial hydatidiform mole is a condition where the fertilized egg contains the normal set of maternal DNAs but double the paternal DNA. Due to this, the embryo only partially develops and becomes an inviable fetus. It normally has a triploid karyotype of 69-XXX, 69-XXY, or 69-XYY; however, a diploid karyotype may exist. A karyotype is the observed characteristics (number, type, shape) of the chromosomes of a fetus.
- Complete hydatidiform mole is a situation of hydatidiform mole where the fertilized egg has no maternal DNA but instead has two sets of paternal DNAs. In this case, a fetus does not form and typically has a karyotyope of 46-XX.
Gestational Trophoblastic Neoplasia
There are about four types of gestational trophoblastic neoplasia. Choriocarcinoma is a cancerous tumor that forms inside the uterus of a pregnant woman. It usually occurs when growths from hydatidiform moles turn cancerous.
Less often, choriocarcinomas form from a tissue left in the uterus after an abortion, miscarriage, delivery of a healthy baby. Placenta-site trophoblastic tumors are not usually discovered until years after a full-term pregnancy.
Epitheliod trophoblastic tumor is an extremely rare tumor’s progression that resembles that of a placenta-site trophoblastic tumor. Invasive mole is a type of GTN where trophoblastic cells form an unusual mass that grows into the muscle layer of the uterus.
Several types of research are ongoing on GTD cells to learn more about how these tumors develop. Revelations about chromosome abnormalities of complete and partial moles have helped explain the etiologies of these types of GTD.
These discoveries have led to developing lab tests that can assist in the diagnosis of the types of moles (whether complete or partial) when routine microscopic analysis does not yield a clear answer.
The gestational trophoblastic disease occurs in about 1 in every 1000 pregnancies in the United States. Most of these are hydatidiform moles.
Choriocarcinoma, the malignant form of Gestational trophoblastic disease (GTD) is even less common, affecting around 2 to 7 of every 100,000 cases in the United States.
Choriocarcinoma and other forms of GTD are more common in many Asian and African countries. Cure rates depend on the type of GTD and its stage.
Malignant Gestational trophoblastic disease stages include:
- Stage I: The cancer growth is still localized in the uterus.
- Stage II: The cancer has spread from the uterus to other structures in the pelvis.
- Stage III: The cancer cell has gotten to the lungs in this stage.
- Stage IV: This is the final stage where the cancer has spread to other organs of the body.
Risk Factors of Gestational Trophoblastic Disease
The gestational trophoblastic disease is a very rare disease and there are some factors that can increase a woman’s risk of developing GTD.
They include:
- History of miscarriage
- Maternal age: If a woman becomes pregnant when she is younger than 20 or older than 35, she has a higher chance of developing the gestational trophoblastic disease.
- Previous molar pregnancy
- Blood type: Specific blood types-A and AB may slightly increase the risk of GTD.
- Family history of molar pregnancy.
- Nutrition/diet: low levels of carotene and vitamin A have been linked to GTD and individuals are at higher risk of molar pregnancy.
Symptoms of Gestational Trophoblastic Disease
Gestational trophoblastic disease symptoms may be associated with many other gynecologic and pregnancy-related conditions. However, one can only know if the symptoms are caused by Gestational trophoblastic disease if evaluated by a doctor (mostly a gynecologist).
A doctor should be consulted if a woman experiences any/all of the following symptoms:
- Bleeding or vaginal discharge not related to a period (menstruation)
- Shortness of breath, fatigue and dizziness due to vaginal bleeding
- High blood pressure
- Headaches and swelling of hands and feet (preeclampsia)
- A larger-than-usual uterus while pregnant
- Pain and/or mass in the pelvic area
- Extreme bouts of nausea and vomiting.
Diagnosis of Gestational Trophoblastic Disease
Diagnosis of a GTD condition will first include an investigation of the medical history of a person and a general physical examination.
One or more of the following tests might be conducted to confirm the diagnosis:
- Pap Smear: This is also known as a pap test and it involves a microscopic examination of cells collected from the cervix, used to detect changes that may be cancer or may lead to cancer and to noncancerous conditions such as inflammation or infections.
- Internal pelvic exam: This is done to feel for any lump or changes in the uterus.
- Blood tests: Doctors use blood samples to check the levels of certain hormones (human chorionic gonadotropin, hCG) produced by trophoblast cells and other substances that may be impacted by the presence of Gestational trophoblastic disease.
- Transvaginal ultrasound: Also referred to as ultrasonography, this ultrasound test uses a small instrument called a transducer, which is placed in the vagina to look at the uterus and nearby tissue.
- Urinalysis: GTD may alter sugar, protein, bacteria, and hCG levels in the urine.
When cancer cells are detected, some subsequent tests are conducted to observe how the disease has spread from the uterus to other parts of the body.
These tests include:
- Spinal tab: The doctor inserts a need into the patient’s spinal column to collect cerebrospinal fluid. This fluid will indicate a high amount of hCG; the test is done when the doctor suspects GTD cells have spread to the brain or spinal cord.
- Computed tomography (CT): Scans of various sections of the abdomen
- Chest X-rays
Differential Diagnosis of Gestational Trophoblastic Disease
Other conditions that may present similar symptoms as Gestational trophoblastic disease include:
- Bladder cancer
- Biliary obstruction
- Brain tumors
- Cerebrovascular accidents
- hCG secreating germ cell tumors
- Nephrolithiasis
- Haemorrhage cystitis: non-infections
- Ovarian choriocarcinoma
- Urothelial tumors of renal pelvis and ureters
- Quieecent GTN
Treatment of Gestational Trophoblastic Disease
The specific treatment approach for Gestational trophoblastic disease is determined by doctors based on the overall health and medical history of the patient, stage and type of GTD, patient’s tolerance for specific medications, procedures and therapies, expectations for the course of the disease, and finally, whether the patient wishes to become pregnant in the future.
Treatment approach may involve:
- Surgical removal of tumors or affected organs.
- Use of anticancer drugs to treat cancerous cells (chemotherapy). In most cases, chemotherapy works by interfering with the cancer cell’s ability to grow. A different group of drugs works in different ways to destroy cancer cells. So, an oncologist will recommend a different treatment plan for different individuals.
- Hysterectomy: This involves the total removal of the uterus. In some cases, it is done with salpingo-oophorectomy, which is a surgery to remove the fallopian tubes and ovaries. Nearby lymph nodes and part of the vagina may also be removed.
- Radiation therapy is sometimes recommended, it uses high energy radiation to kill cancer cells and shrink tumors.
There are no sure ways of preventing Gestational trophoblastic disease and no preventive medication or treatment for GTD exist. The only way to prevent this very rare pregnancy complication is not to get pregnant.
Doctors on patients’ treatment team may include:
- A gynecologist: a doctor who specializes in the disease of the female reproductive system.
- A gynecologic oncologist: a doctor who specializes in cancers of the female reproductive system.
- A radiation oncologist: a doctor who uses radiation to treat cancer.
- A medical oncologist: a doctor who uses chemotherapy and other medicines to treat cancer.
Many other specialists may be involved in a patient’s care as well, including nurses, psychologist, social workers and rehabilitation specialists.
Summary
Completing treatment may be psychologically and physically strenuous and exciting. Most individuals fear recurrence of the tumor. This is a very common concern in people who have had either a benign or malignant tumor.
The risk of recurrence of Gestational trophoblastic disease is very small for molar pregnancies and low risk GTD, but it might be as high as 15% in women with high-risk GTD.
For this cause, medical follow-up plans are very important. Talk with your physician about developing a survivorship plan, if so concerned.
These plans might entail:
- A suggested schedule for follow-up exams and tests
- A schedule for other tests a person might need in the future, or tests to look for long-term health effects from the condition or its treatment.
- Diet and physical activity suggestions
- A list of possible late- or long-term side effects from previous treatment
- Reminders to keep appointments with a primary care provider (PCP), who will monitor a patient’s general health care.
Sources
- Gestational Trophoblastic Disease; http://www.webmd.com/cancer/gestational_trophoblastic_disease
- Gestational Trophoblastic Disease; https://teachmeobgyn.com/pregnancy/early/gestational-trophoblastic-disease/
- Gestational Trophoblastic Disease; http://www.foundationforwomenscancer.org/glynecologic-cancers/cancer-typers/gestational-trophoblastic/
- Gestational Trophoblastic Disease; https://www.bcm.edu/healthcare/specialties/womens-health-maternity/obgyn-conditions/gestational-trophoblastic-disease